MONJUVI, in combination with lenalidomide, was granted accelerated approval based on the 1-year primary analysis of the L-MIND study.
1-year primary analysis in patients with R/R DLBCL (N=71)1*:
-
Best ORR: 55% (n=39; 95% CI: 43%, 67%); CR: 37%; PR: 18%
-
Median DoR: 21.7 months (range: 0, 24)†
Review the 1-year primary analysis data here.
I’m very pleased to have a treatment option like MONJUVI with 5-year follow-up data for my patients.
— Dr Hayder Saeed


Watch DLBCL expert Dr Hayder Saeed discuss MONJUVI 5-year data
Today I want to talk about 5-year follow-up data for MONJUVI, an outpatient targeted immunotherapy given in combination with lenalidomide as a second- or later-line therapy for adult non-transplant eligible patients with relapsed or refractory diffuse large B-cell lymphoma, or DLBCL. The 5-year analysis data from L-MIND have not been submitted to or reviewed by the FDA, and potential inclusion of these data in the final FDA-approved labeling has yet to be determined. I am Dr Hayder Saeed, an Associate Member at the Moffitt Cancer Center. Before reviewing the efficacy data for L-MIND, it’s important to review the full indication and some brief safety information first.
MONJUVI (tafasitamab-cxix), in combination with lenalidomide, is indicated for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) not otherwise specified, including DLBCL arising from low grade lymphoma, and who are not eligible for autologous stem cell transplant, or ASCT.
This indication is approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
Warnings and Precautions
Infusion-Related Reactions (IRRs)
MONJUVI can cause infusion-related reactions (IRRs). In L-MIND, infusion-related reactions occurred in 6% of the 81 patients. Eighty percent of infusion-related reactions occurred during cycle 1 or 2. Signs and symptoms included fever, chills, rash, flushing, dyspnea, and hypertension. These reactions were managed with temporary interruption of the infusion and/or with supportive medication. Premedicate patients prior to starting MONJUVI infusion. Monitor patients frequently during infusion. Based on the severity of the infusion-related reaction, interrupt or discontinue MONJUVI. Institute appropriate medical management.
Now let’s discuss the efficacy of MONJUVI, which is the only second-line, outpatient targeted immunotherapy for non-transplant eligible patients with relapsed or refractory DLBCL with 5-year data available. The pivotal trial, L-MIND, was an open-label, multicenter, single-arm, Phase 2 trial that included patients with relapsed or refractory DLBCL after 1 to 3 prior systemic DLBCL therapies, including a CD20-containing therapy, and who were ineligible for or refused autologous stem cell transplant. Efficacy was established in 71 patients based on best overall response rate, or ORR (defined as the proportion of complete and partial responders), and duration of response, or DoR, as assessed by an Independent Review Committee using the Cheson 2007 International Working Group Response Criteria.
MONJUVI was administered in combination with lenalidomide for a maximum of 12 cycles, then as a monotherapy until disease progression or unacceptable toxicity. It’s important to note that the trial included patients who have characteristics traditionally considered difficult to treat. 19.7% of patients were primary refractory, and 52.1% had an IPI score of 3-5. In the 1-year primary analysis, the best ORR was 55%, with a complete response rate of 37% and a partial response rate of 18%. The median DoR was 21.7 months. In terms of how long it took patients to respond the median time to response was 2 months. Note that the time-to-response analysis is exploratory in nature. The time-to-response data should be interpreted with caution due to single-arm studies not adequately characterizing time-to-event endpoints, and the small sample size, which may lead to estimates that are unstable. Let’s briefly review some more safety information before continuing.
Myelosuppression
MONJUVI can cause serious or severe myelosuppression, including neutropenia, thrombocytopenia, and anemia. In L-MIND, Grade 3 neutropenia occurred in 25% of patients, thrombocytopenia in 12%, and anemia in 7%. Grade 4 neutropenia occurred in 25% and thrombocytopenia in 6%. Neutropenia led to treatment discontinuation in 3.7% of patients. Monitor complete blood counts (CBC) prior to administration of each treatment cycle and throughout treatment. Monitor patients with neutropenia for signs of infection. Consider granulocyte colony-stimulating factor (G-CSF) administration. Withhold MONJUVI based on the severity of the adverse reaction. Refer to the lenalidomide prescribing information for dosage modifications.
Infections
Fatal and serious infections, including opportunistic infections, occurred in patients during treatment with MONJUVI and following the last dose. In L-MIND, 73% of the 81 patients developed an infection. The most frequent infections were respiratory tract infection (24%), urinary tract infection (17%), bronchitis (16%), nasopharyngitis (10%) and pneumonia (10%). Grade 3 or higher infection occurred in 30% of the 81 patients. The most frequent grade 3 or higher infection was pneumonia (7%). Infection-related deaths were reported in 2.5% of the 81 patients. Monitor patients for signs and symptoms of infection and manage infections as appropriate.
Embryo-Fetal Toxicity
Based on its mechanism of action, MONJUVI may cause fetal B-cell depletion when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise women of reproductive potential to use effective contraception during treatment with MONJUVI and for at least 3 months after the last dose. MONJUVI is initially administered in combination with lenalidomide. The combination of MONJUVI with lenalidomide is contraindicated in pregnant women because lenalidomide can cause birth defects and death of the unborn child. Refer to the lenalidomide prescribing information on use during pregnancy.
Now let’s discuss the 5-year, long-term data. The ORR held steady at 54%, with a complete response rate of 37% and a partial response rate of 17%. It’s also interesting to see the efficacy outcomes broken down by those who received MONJUVI in the second line versus the third-line plus setting. As you can see, in the exploratory subgroup analysis shown here, 89% more patients achieved a complete response in second line versus third line plus. Note that L-MIND was not designed or powered to evaluate and compare multiple subgroups. These data should be interpreted with caution due to the study design characteristics described earlier. With a median follow-up of 54 months, or 4.5 years, the median DoR was not reached, demonstrating a sustained response with MONJUVI. Approximately 57% of responding patients were still in remission at 5 years.
The FDA approval of MONJUVI in 2020 provided an important treatment option for appropriate patients with R/R DLBCL, and it is estimated that more than 4,000 patients have been treated in the US since approval. I’m very pleased to have a treatment option like MONJUVI with 5-year follow-up data for my patients. Before closing, please stay tuned for additional important safety information for MONJUVI.
Adverse Reactions:
Serious adverse reactions occurred in 52% of patients who received MONJUVI. Serious adverse reactions in ≥6% of patients included infections (26%), including pneumonia (7%), and febrile neutropenia (6%). Fatal adverse reactions occurred in 5% of patients who received MONJUVI, including cerebrovascular accident (1.2%), respiratory failure (1.2%), progressive multifocal leukoencephalopathy (1.2%) and sudden death (1.2%).Permanent discontinuation of MONJUVI or lenalidomide due to an adverse reaction occurred in 25% of patients and permanent discontinuation of MONJUVI due to an adverse reaction occurred in 15%. The most frequent adverse reactions which resulted in permanent discontinuation of MONJUVI were infections (5%), nervous system disorders (2.5%), respiratory, thoracic and mediastinal disorders (2.5%).
Dosage interruptions of MONJUVI or lenalidomide due to an adverse reaction occurred in 69% of patients and dosage interruption of MONJUVI due to an adverse reaction occurred in 65%. The most frequent adverse reactions which required a dosage interruption of MONJUVI were blood and lymphatic system disorders (41%), and infections (27%). The most common adverse reactions (≥20%) were neutropenia (51%), fatigue (38%), anemia (36%), diarrhea (36%), thrombocytopenia (31%), cough (26%), pyrexia (24%), peripheral edema (24%), respiratory tract infection (24%), and decreased appetite (22%).
Please see the full Prescribing Information available at MONJUVIhcp.com
The 5-year analysis data from L-MIND have not been submitted to or reviewed by the FDA, and potential inclusion of these data in the final FDA-approved labeling has yet to be determined. This disclaimer applies to all data from the 5-year analysis below.
5-year ORR analysis2*
ORR in patients with R/R DLBCL (N=71)
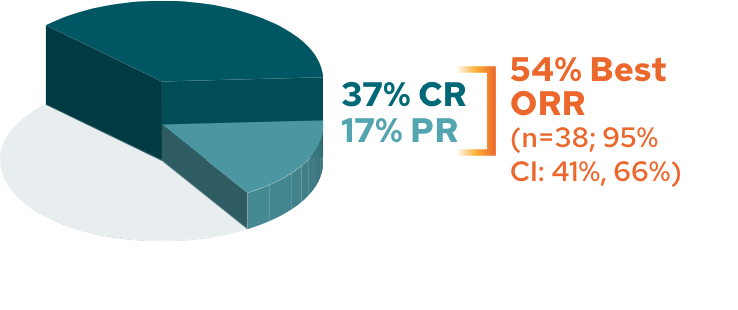
The cutoff date for the 5-year follow-up analysis was November 14, 2022, and occurred after the last patient enrolled had completed 5 years of follow-up2
Response rates in 2L and 3L+2*
Response rates by line of therapy in 5-year analysis
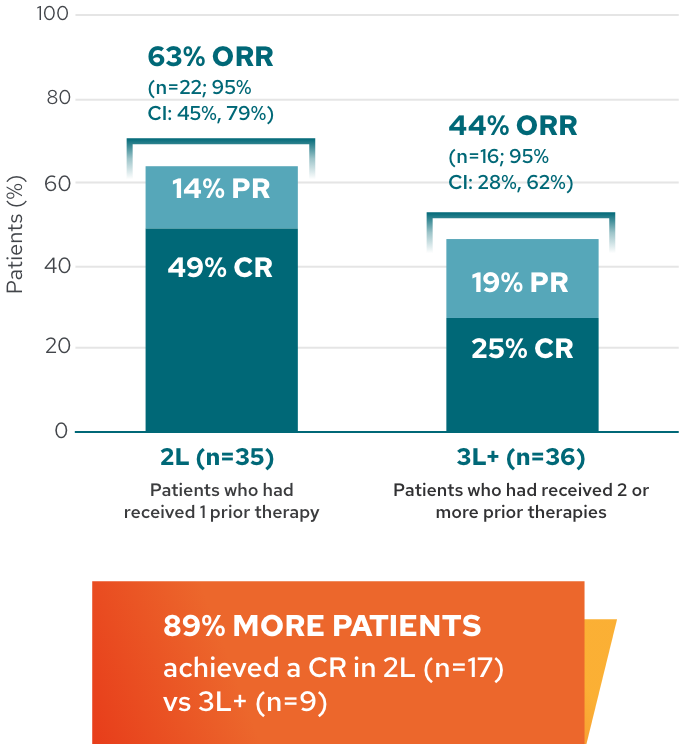
This analysis is exploratory in nature, and L-MIND was not designed or powered to evaluate and compare multiple subgroups. These results should be interpreted with caution given the small sample size, which may lead to estimates that are unstable.
Median DoR in patients with R/R DLBCL2,3
5-year follow-up analysis: median DoR not reached*†
(median follow-up 53.8 months [95% CI: 31.8-58.7])
![L-MIND duration of response Kaplan-Meier graph. A 5-year follow-up analysis in patients with R/R DLCBL (N=71). Median DoR not reached (median follow-up 53.8 months [95% CI: 31.8-58.7]).](/images/efficacy/median-mobile.png)
*Assessed by an Independent Review Committee.1,3
†Kaplan-Meier estimates.1,3
‡DoR rate at 5 years is a Kaplan-Meier estimate and should be interpreted with caution due to the small sample size and the number of censored patients.
The cutoff date for the 5-year follow-up analysis was November 14, 2022, and occurred after the last patient enrolled had completed 5 years of follow-up.2
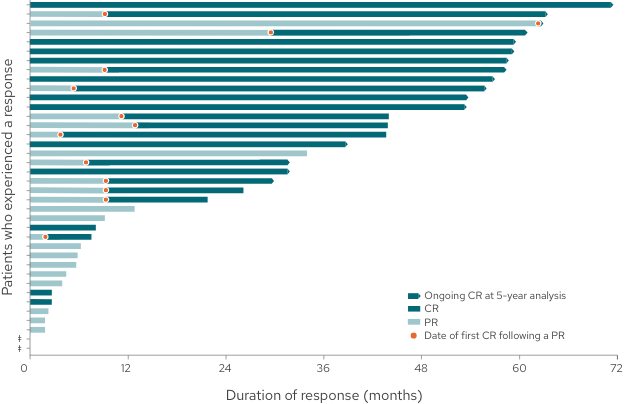
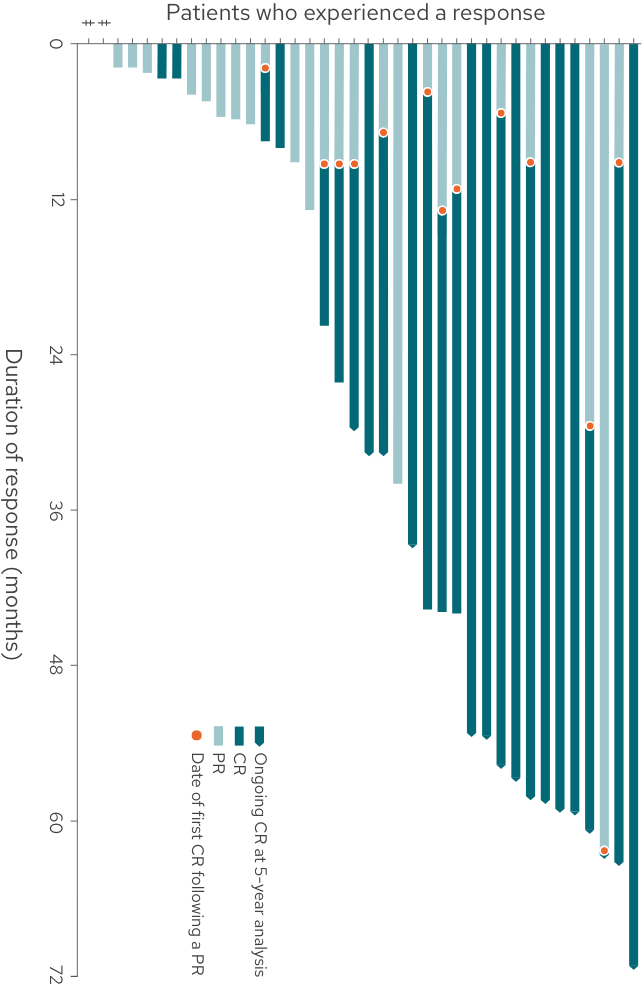
Duration of response by patient3
DoR by patient from time of initial response3
 +
+
 Tap to
enlarge
Tap to
enlarge
-
68% of responding patients (26/38) achieved a best response of CR
-
34% of responders (13/38) converted from a PR to a CR
-
32% of responding patients (12/38) had a response of at least 4 years
This graph and these bullets represent 38 out of the 71 patients in the L-MIND study who experienced a response; 33 patients did not experience a complete or partial response.
For patients whose response has stopped, events may include tumor progression, death, or beginning of a new anti-cancer treatment.
The study concluded on the 5-year analysis cutoff date of November 14, 2022.3
This analysis is exploratory in nature. These results should be interpreted with caution due to single-arm studies not adequately characterizing time-to-event endpoints, and the small sample size, which may lead to estimates that are unstable.
This analysis is exploratory in nature. These results should be interpreted with caution due to single-arm studies not adequately characterizing time-to-event endpoints, and the small sample size, which may lead to estimates that are unstable.
§Two patients started a new anti-cancer treatment immediately after a response was recorded at the end of the treatment assessment.3
The initial assessment of efficacy/disease response was performed and recorded at Cycle 3, Day 1.4
The cutoff date for the 5-year follow-up analysis was November 14, 2022, and occurred after the last patient enrolled had completed 5 years of follow-up.3
L-MIND study design
-
L-MIND was an open-label, multicenter, single-arm, Phase 2 study in adult patients with R/R DLBCL who were not eligible for or refused ASCT.1,5
-
Efficacy was established based on best ORR (defined as the proportion of complete and partial responders), and DoR, as assessed by an Independent Review Committee using the International Working Group Response Criteria (Cheson 2007).1
See the full study design here.

